INTERMOLECULAR BONDING - HYDROGEN BONDS
The evidence for hydrogen bonding
Many elements form compounds with hydrogen. If you plot the
boiling points of the compounds of the Group 4 elements with hydrogen, you find
that the boiling points increase as you go down the group.
The increase in boiling point happens because the molecules
are getting larger with more electrons, and so van der Waals dispersion forces
become greater.
Note: If you aren't
sure about van der Waals dispersion forces, it would pay you to follow this
link before you go on.
If you repeat this exercise with the compounds of the
elements in Groups 5, 6 and 7 with hydrogen, something odd happens.
Although for the most part, the trend is the same as in
group 4 (for the same reasons), the boiling point of the compound of hydrogen
with the first element in each group is abnormally high.
In the cases of NH3, H2O, and HF there must be some
additional intermolecular forces of attraction, requiring significantly more
heat energy to break. These relatively powerful intermolecular forces are
described as hydrogen bonds.
The origin of hydrogen
bonding
The molecules which have this extra bonding are:
Note: The solid line
represents a bond in the plane of the screen or paper. Dotted bonds are going
back into the screen or paper away from you, and wedge-shaped ones are coming
out towards you.
Notice that in each of these molecules:
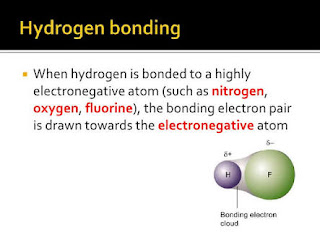
The hydrogen is attached directly to one of the most
electronegative elements, causing the hydrogen to acquire a significant amount
of positive charge.
Each of the elements to which the hydrogen is attached is
not only significantly negative but also has at least one "active" lone
pair.
Lone pairs at the 2-level have the electrons contained in a
relatively small volume of space which therefore has a high density of negative
charge. Lone pairs at higher levels are more diffuse and not so attractive to
positive things.
Note: If you aren't
happy about electronegativity, you should follow this link before you go on.
Consider two water molecules coming close together.
The δ+ hydrogen is so strongly attracted to the lone pair
that it is almost as if you were beginning to form a co-ordinate (dative
covalent) bond. It doesn't go that far, but the attraction is significantly
stronger than an ordinary dipole-dipole interaction.
Hydrogen bonds have about a tenth of the strength of an
average covalent bond and are being broken continuously and reformed in liquid
water. If you liken the covalent bond between the oxygen and hydrogen to a
stable marriage, the hydrogen bond has "just good friends" status.
Water as a "perfect" example of hydrogen bonding
Notice that each water molecule can potentially form four
hydrogen bonds with surrounding water molecules. There are exactly the right
numbers of δ+ hydrogens and lone pairs so that every one of them can be
involved in hydrogen bonding.
This is why the boiling point of water is higher than that
of ammonia or hydrogen fluoride.
Note: You will find
more discussion on the effect of hydrogen bonding on the properties of water in
the page on molecular structures.
In the case of ammonia, the amount of hydrogen bonding is
limited by the fact that each nitrogen only has one lone pair. In a group of
ammonia molecules, there aren't enough lone pairs to go around to satisfy all
the hydrogens.
That means that on average each ammonia molecule can form
one hydrogen bond using its lone pair and one involving one of its δ+ hydrogens.
The other hydrogens are wasted.
In hydrogen fluoride, the problem is a shortage of hydrogens.
On average, then, each molecule can only form one hydrogen bond using its δ+ hydrogen
and one involving one of its lone pairs. The other lone pairs are mostly wasted.
In water, there is precisely the right number of each. Water
could be considered as the "perfect" hydrogen bonded system.
Warning: It has been
pointed out to me that some sources (including one of the UK A level Exam
Boards) count the number of hydrogen bonds formed by water, say, differently. They
say that water forms 2 hydrogen bonds, not 4. That is often accompanied by a
diagram of ice next to this statement clearly showing 4 hydrogen bonds!
Reading what they say, it appears that they only count a
hydrogen bond as belonging to a particular molecule if it comes from a hydrogen
atom on that molecule. That seems to me to be illogical. A hydrogen bond is
made from two parts - a δ+ hydrogen attached to a sufficiently electronegative
element, and an active lone pair. These interact to make a hydrogen bond, and
it is still a hydrogen bond irrespective of which end you look at it from.
The IUPAC definitions of a hydrogen bond do not refer at all
to any of this, so there doesn't seem to be any "official" backing
for this one way or the other.
However, it is essential that you find out what your
examiners are expecting. They make the rules for the exam you will be sitting, and
you have no choice other than to play by those rules.